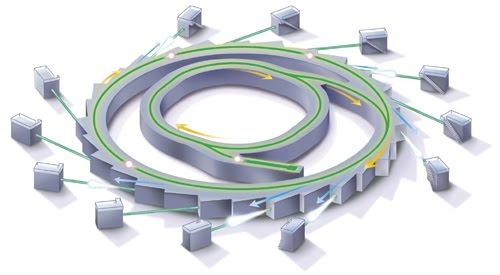
1. The Linac consists of an electron gun to send electrons into a vacuum and then uses radio-frequency electromagnetic waves to accelerate the electrons to high speed.
Electrons escape from the electron gun as they are boiled off the heated solid. They are then accelerated across a potential difference of about 100kev and exit the gun at a speed of about 50% the speed of light. The electron beam travels through an ultra-high vacuum within the linac to prevent energy loss through collisions with air particles. Then radio-frequency electromagnetic waves accelerate the electrons, energising them to a kinetic energy of 100MeV.
2. The Booster Ring: within the circular booster ring, bending magnets provide a force at right angles to the motion of the electrons in order to bend them in a circular path. The electrons' energy increases to 3000MeV (3 GeV or 3 gigaelectronvolts). The energy boost is supplied by a radio-frequency chamber through which the electrons travel on each orbit of the ring.
Rather than gaining extra speed, the electrons are only getting slightly faster as they are already near the speed of light. They have increased in mass by a factor of 6000 times their rest mass.
The radius of curvature of their bending path is given by
r = mv/(eB)
such that as the radio-frequency booster gives the electrons more energy and their mass increases, then the beam radius increases which would result in the electrons hitting the wall of the evacuated tube. To prevent this happening, the strength of the magnetic field (B) is increased at the same rate as the energy (and hence mass) increases.
3. The Storage Ring: The booster ring transfers electrons into the storage ring, which has a radius of 34 m and circumference of 216 m. In one second the electrons make over one million revolutions of the storage ring and continue to circulate for between 5 and 50 hours.
As the electron path bends, synchrotron radiation is given off tangentially to the path. The energy lost to the electrons in giving off the radiation is replenished by a radio-frequency boost each revolution, so that the electrons maintain the same energy. Unused high energy X-rays given off by the storage ring are continually absorbed by concrete radiation shielding. This tunnel completely encloses the storage ring, except for narrow openings providing the beamlines through which the synchrotron X-ray radiation is guided.
4. A beamline is the path that the synchrotron light travels from the storage ring to its target experimental work in the optics room. A beamline is a stainless steel tube, 15-35 m in length and around 4 cm in diameter.
If scientists only want a specific range of wavelengths for their experiments, a device called a monochromator (a crystal or a grating) is used as a wavelength selector. As the beam hits the monochromator, a specific light frequency can be selected from the broad band of frequencies in the incident beam. Devices called attenuators can be used to reduce the intensity of the beam if required.
Scientists monitor their experiments at a work station using a computer in an external control room, in which they are protected from the intense electromagnetic radiation being used in the experiments.
A great benefit of synchrotron light sources is that virtually any radiation wavelength can be used in experiments requiring radiation wavelengths from infrared to X-ray.
5. Insertion Devices: Dipole Magnets, Wigglers and Undulators can be inserted in straight sections of the storage ring.
The dipole magnets in the storage ring which are used to bend the beam are a source of synchrotron light.
Stronger sources of synchrotron light are wigglers and undulators. They are not essential to the operation of the storage ring, their only function is to create more intense synchrotron light.
A wiggler consists of two rows of small alternating magnetic poles, which force the elctrons into a wavy path (the electrons "wiggle" along the path).
An undulator consists of less powerful magnets than that of the wiggler, producing gentler deflections of the electron beam. The emitted synchrotron light overlaps to produce a beam that is collimated to a narrow beam.
The undulator insertion device results in interference effects that produce a spectrum of synchrotron light that is enhanced at specific wavelengths. These wavelengths are determined by the spacing between magnetic poles of the undulator, and can be mechanically tuned to select wavelengths. For the wavelengths produced, the brightness can be one million times that produced by the ending dipole magnets.
Synchrotron Light compared to X-ray tubes and Lasers
The X-ray light produced by a Synchrotron is much brighter and intense than X-rays produced by an X-ray tube (100 million times brighters).
Synchrotron light compared to Laser light. Lasers produce light of one wavelength only, so that you need many different individual lasers for different wavelengths. The power of a laser is very weak compared to that of the synchrotron. Lasers are limited to infrared and visible wavelengths. Some lasers can produce ultraviolet light. Bothy the synchrotron and Lasers can produce coherent (in phase) light. Normal lasers cannot produce X-ray light. Synchrotrons can produce laser light in the X-ray region using an undulator.
Monday, September 6, 2010
Monday, August 16, 2010
De Broglie's Wave Theory for Electron Energy Levels in the Atom
To explain the Bohr Atom Energy Levels for the Hydrogen Atom, De Broglie postulated that an electron orbiting the proton nucleus of the Hydrogen Atom would only occupy discrete orbits due to the wave nature of the electron. What de Broglie hypothesised was that for each orbit, the circumference could only be of a length equal to an integral number of wavelengths.
De Broglie was theorising that each orbit corresponded to a whole number of wavelengths.
The ground state should have a circumference of one electron wavelength: n = 1.
The first excited state would have two electron wavelengths fitted into it: n = 2.
The second excited state would have a length equal to three electron wavelengths: n = 3: and so on.
De Broglie was drawing on the understanding of sound waves and musical instruments such as stringed instruments, where it was known that standing waves are set up when a stringed instrument vibrates. Each octave in the musical scale corresponds to an integral number of wavelengths in a standing wave.
Einstein was interested in de Broglie's postulate and asked Schrodinger to analyse it from a mathematical perspective.
Schrodinger then postulated that an electron has a wave function and developed an equation from which he established a solution.
Developing a solution to his equation was easiest for a simple atom such as the Hydrogen atom. Schrodinger's solution showed remarkable consistency with the Bohr atom energy levels.
The energy levels of hydrogen, as calculated by Schrodinger are given by
where n = 1 for the ground state, n=2 is the first excited state, n = 3 is the second excited state etc.
Saturday, August 14, 2010
Hydrogen Emission and Absorption Spectrum
The Hydrogen Emission Spectrum consists of a series of coloured lines resulting from the transition of its electron from one energy level down to another; a photon being emitted with its energy equal to the difference between the energy levels.
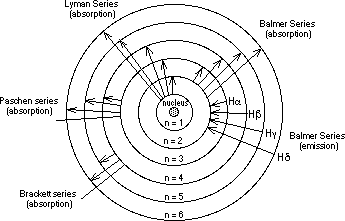
There are many clusters of lines in the Hydrogen spectrum. Only one is in the visible region: the Balmer series, named after the man who worked out the mathematical formula for it. As a result of his mathematical analysis of the wavelengths of the visible hydrogen lines; he predicted many more which were later found by experiment in the ultraviolet region. Rydberg generalised Balmer's formula and then predicted other series of spectral lines which were subsequently observed by experiments: (the Lyman series) in the ultraviolet, and in the infrared region ( the Paschen series and the Brackett Series) and well as others in the far infrared etc.
The Energy Levels of the Hydrogen Atom:
The Transitions between Energy Levels of the Electron either results from absorption of a photon of the required energy or emission of a photon of that same energy. The chart below shows many of the possible emissions due to electrons falling from one excited state to a lower energy state.
The absorption spectrum for Hydrogen, arises when we view white light coming through hydrogen gas, as is typically observed by astronomers when they analyse the light coming from distant stars; the light from those stars passing through clouds of cold hydrogen gas. The hydrogen atoms in the clouds absorb light of the frequency matching the difference in energy levels of the hydrogen atom, raising an electron up a level or number of levels. This depletes the light coming from the star for these particular wavelengths, thus creating dark lines in a continuous spectrum.
There are many clusters of lines in the Hydrogen spectrum. Only one is in the visible region: the Balmer series, named after the man who worked out the mathematical formula for it. As a result of his mathematical analysis of the wavelengths of the visible hydrogen lines; he predicted many more which were later found by experiment in the ultraviolet region. Rydberg generalised Balmer's formula and then predicted other series of spectral lines which were subsequently observed by experiments: (the Lyman series) in the ultraviolet, and in the infrared region ( the Paschen series and the Brackett Series) and well as others in the far infrared etc.
The Energy Levels of the Hydrogen Atom:
The Transitions between Energy Levels of the Electron either results from absorption of a photon of the required energy or emission of a photon of that same energy. The chart below shows many of the possible emissions due to electrons falling from one excited state to a lower energy state.
The absorption spectrum for Hydrogen, arises when we view white light coming through hydrogen gas, as is typically observed by astronomers when they analyse the light coming from distant stars; the light from those stars passing through clouds of cold hydrogen gas. The hydrogen atoms in the clouds absorb light of the frequency matching the difference in energy levels of the hydrogen atom, raising an electron up a level or number of levels. This depletes the light coming from the star for these particular wavelengths, thus creating dark lines in a continuous spectrum.
Saturday, August 7, 2010
Bohr Model of the Atom
White light has a continuous spectrum of colours.
Fraunhofer observed dark lines in the light spectrum of the sun.
The emission and absorption spectra of atoms such as Hydrogen differ from the continuous spectrum of white light.
After Fraunhofer, astronomers observed absorption spectra in all types of stars.
Neils Bohr realised that emission and absorption spectra were an indicator for how the electrons in an atom behaved. The observed spectra gave a clue to the structure of the atom.
(1) absorption spectra showed that atoms such as the hydrogen atom were only capable of absorbing a small number of different frequencies, and therefore energies.

(2) the emission spectra of hydrogen showed that hydrogen was also capable of emitting photons of the exact energy value that it was able to absorb.
Bohr proposed that:
(1) a number of allowable electron orbits of different radii exist for each atom labeled n=1,2,3,... And the electron may occupy only these orbits.
(2) An electron ordinarily occupies the lowest energy orbit available. In this orbit of lowest energy, it does not radiate energy.
(3) Electromagnetic radiation can be
 absorbed by an atom when its photon energy is exactly equal to the difference in energies between an occupied orbit and a higher energy orbit. This photon absorption results in an excited atom.
absorbed by an atom when its photon energy is exactly equal to the difference in energies between an occupied orbit and a higher energy orbit. This photon absorption results in an excited atom.(4) Electromagnetic radiation is emitted by an excited atom when an electron falls from a higher energy level to a lower energy level. The photon energy will be exactly equal to the energy difference between the electron's initial and final level.
Matter Waves

De Broglie postulated that matter such as electrons should exhibit the wave particle duality just as light does.
He theorised that electrons would have a wavelength inversely proportional to its momentum, and that its kinetic energy would be directly proportional to its frequency.
The observation of electron diffraction patterns, similar to that of x-rays (where each have equivalent wavelengths) provided experimental confirmation of De Broglie's theoretical prediction.
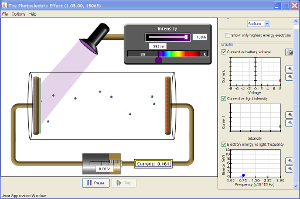
Photoelectric Effect Applet
Photoelectric effect
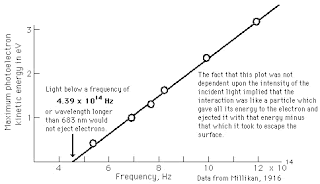 The photoelectric effect is a phenomenon in which electrons are emitted from matter (metals and non-metallic solids, liquids or gases) as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light.
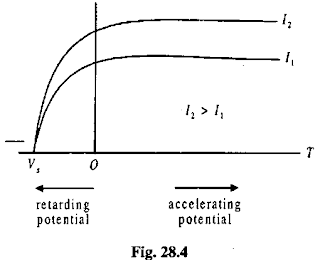
The photoelectric effect is a phenomenon in which electrons are emitted from matter (metals and non-metallic solids, liquids or gases) as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light.In 1902, Philipp Lenard observed that the energy of individual emitted electrons increased with the frequency of the light. This appeared to be at odds with Maxwell's wave theory of light, which was thought to predict that the electron energy would be proportional to the intensity of the radiation.
When Lenard applied a retarding potential to the electrons he found that the 'stopping voltage' depended both on the frequency of the light and the material of the electrode, but did not depend on the intensity of the light. Brighter light did not increase the kinetic energy of the electrons.
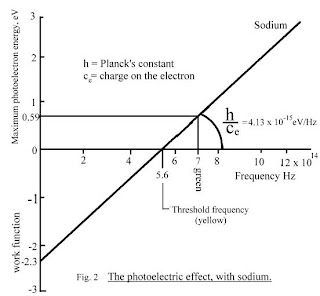 In 1905, Einstein solved this apparent paradox by describing light as composed of discrete quanta, now called photons, rather than continuous waves. Einstein theorized that the energy in each quantum of light was equal to the frequency multiplied by a constant, later called Planck's constant.
In 1905, Einstein solved this apparent paradox by describing light as composed of discrete quanta, now called photons, rather than continuous waves. Einstein theorized that the energy in each quantum of light was equal to the frequency multiplied by a constant, later called Planck's constant.E = hf - W
where E = electron kinetic energy
f = light frequency
and W = work function
The Work Function is the energy required to eject the electron.
W = hf0 where f0 is the threshold frequency.
Young's Double Slit Experiment
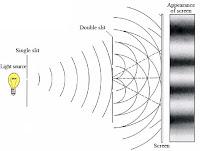
A light source (with a narrow band of wavelengths eg red light) illuminates a thin plate with two parallel slits cut in it, and the red light passing through the slits strikes a screen behind them. The wave nature of light causes the light waves passing through both slits to interfere, creating an interference pattern of bright red and dark bands on the screen.
View the Walter Fendt applett at
http://www.walter-fendt.de/ph14e/doubleslit.htm
Subscribe to:
Posts (Atom)